Artificial intelligence and machine learning technologies are poised to transform healthcare sooner, rather than later, if the FDA’s increased interest is any indication. Starting with the 21st Century Cures Act and subsequent draft guidance aimed at lessening the regulatory burden for some clinical decision support software, the FDA has its eyes on paving a clearer path to accelerate medical product development and bringing healthcare innovations and advances to patients who need them faster and more efficiently .
This year, the regulatory body has granted approvals to several machine learning tools, including medical imaging software that can help detect strokes and another that can spot and diagnose wrist fractures.
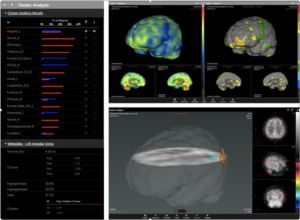
In February, we received FDA 510(k) clearance for our neuroimaging analytics and quantification platform, CereMetrix SilverTM. When we started the FDA process in late 2017, we had concerns about it becoming a daunting task. However, it was a surprisingly positive experience for us. We were provided direct access to our FDA reviewer who guided us every step of the way and helped us receive clearance in five months.
The FDA has continued to make concerted efforts in streamlining the approval process for AI- and machine learning based devices and tools. In April, the regulatory body announced plans to expand its regulatory coverage to encourage and prepare for the development of health products that incorporate AI and machine learning technologies. The FDA is working with industry experts to update the way it evaluates AI-based healthcare technologies by considering how it can add the segment to its pre-certification program.
In May, the FDA proposed reclassifying computer-aided detection devices used in mammography for breast cancer, ultrasound for breast lesions, x-ray for lung nodules and x-ray for dental caries as class II medical devices instead of class III. If approved, the proposal would remove the requirement to submit a premarket approval application and impose a less burdensome premarket notification (510(k)) process before marketing a device. Most recently, the FDA issued an update to its software pre-certification program, requesting public comment.
The recent approvals and proposals by the FDA to reduce regulatory burdens on machine learning healthcare innovations signal the start of a new era in healthcare. With FDA 510(k) clearance for CereMetrix SilverTM in hand and more FDA filings planned for CereMetrix in the near future, we are ecstatic to play a role in AI’s positive disruption of healthcare.
If you would like to learn more about CereMetrix®, I will be at the 2018 Society of Nuclear Medicine and Molecular Imaging (SNMMI) Annual Meeting in Philadelphia on June 24 – 26. To schedule a time to meet, please visit Booking.CereMetrix.io.
John Kelley
President of CereMetrix Corp.